Marchio Comunitario Depositato n° 001240407 presso UAMI - Alicante - Spagna La sottoscritta NERI S.P.A. Via 8 Marzo, 6 - 42025
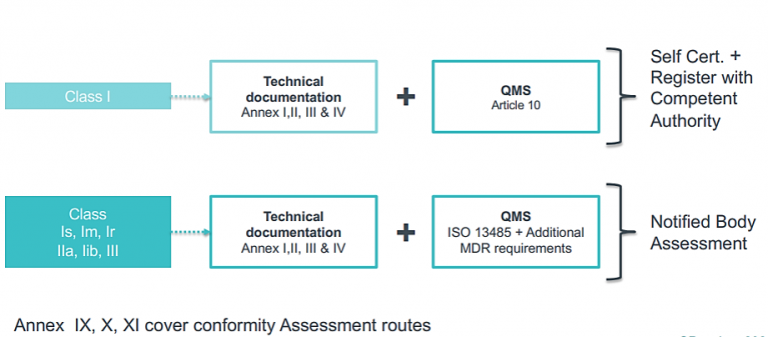
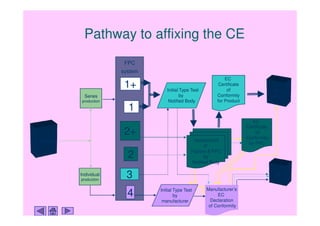
CE Marking a Medical Device under the EU MDR | Wellcome / EPSRC Centre for Interventional and Surgical Sciences - UCL – University College London